News Center
Contribute to the cause of human health
Authoritative release! 41 hospitals in Henan were notified and 69 companies were rectified
- 2020-07-15
- Views:
(Summary description)Recently , the official website of the Henan Provincial Drug Administration announced a notice on the inspection and rectification of medical devices in the second half of 2019, involving medical device operators and users. 41 hospitals were rectified, and color Doppler ultrasound and ventilators were the focus of inspection.
Authoritative release! 41 hospitals in Henan were notified and 69 companies were rectified
(Summary description)Recently , the official website of the Henan Provincial Drug Administration announced a notice on the inspection and rectification of medical devices in the second half of 2019, involving medical device operators and users. 41 hospitals were rectified, and color Doppler ultrasound and ventilators were the focus of inspection.
- Industry news
- 2020-07-15 15:15
- Views:
Recently, the official website of the Henan Provincial Drug Administration announced a notice on the inspection and rectification of medical devices in the second half of 2019, involving medical device operators and users. 41 hospitals were rectified , and color Doppler ultrasound and ventilators were the focus of inspection .
The full text is as follows :
Announcement of Henan Provincial Drug Administration on the rectification of unannounced inspections of medical device users in the second half of 2019

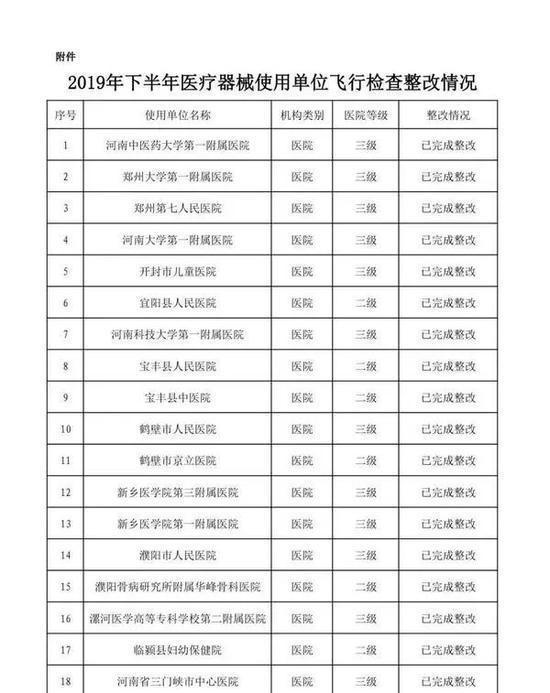
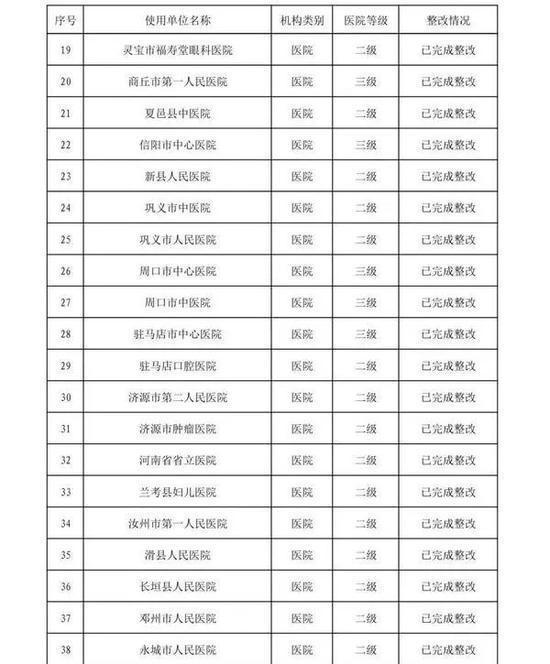
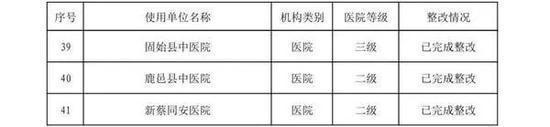
In the second half of 2019, in accordance with the "Measures for Unannounced Inspection of Pharmaceuticals and Medical Devices", the Henan Provincial Drug Administration organized a total of 41 unannounced inspections of medical device users.
Develop inspections for key products such as high-value medical consumables, sterile and implants, in vitro diagnostic reagents, sodium hyaluronate for injection, customized dentures, color ultrasound diagnostic equipment, perfluoropropane gas, infant incubators, ventilators, etc. The plan, in accordance with the requirements of the medical device use quality supervision and management measures, focuses on inspecting the implementation of the main responsibility for the quality and safety of the use of medical devices, the quality and safety management of the use of medical devices, and the monitoring of procurement, acceptance, storage, use, maintenance, and adverse event monitoring. A comprehensive inspection was conducted on key links. The inspection found that, to varying degrees, the user units had problems such as incomplete medical device quality management systems, inadequate implementation, inadequate procurement and acceptance management, and irregular documentation.
For the problems and clues found in the inspection, the inspection team has been transferred to the local supervisory department of the medical device user in accordance with the "Regulations on the Supervision and Administration of Medical Devices", "Administrative Measures on the Monitoring and Re-evaluation of Medical Device Adverse Events", "Administrative Measures on the Quality Supervision and Administration of Medical Device Use" Relevant regulations shall conduct investigation and handling according to law. At the same time, the user units are urged to rectify existing problems.
The rectification of unannounced inspections of medical device users in the second half of 2019 (see attachment) is hereby notified.
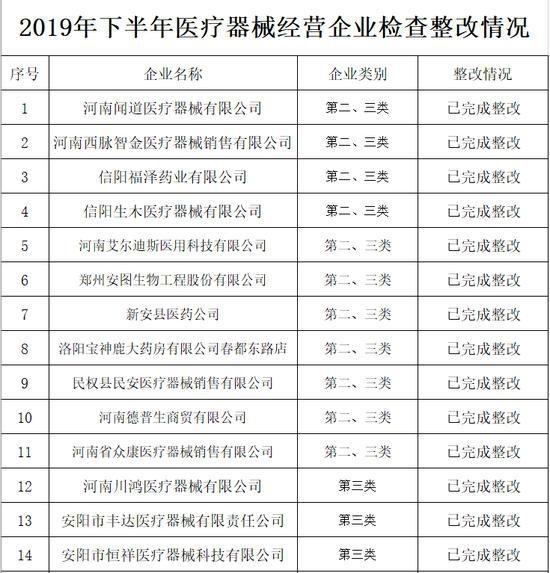
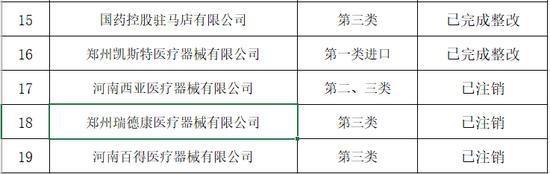
Announcement of Henan Provincial Drug Administration on the rectification of unannounced inspections of medical device operating companies in the second half of 2019
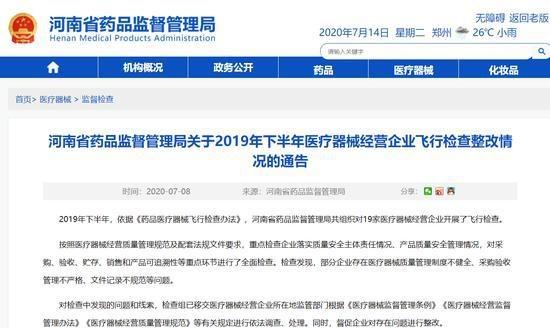
In the second half of 2019, in accordance with the "Measures for Unannounced Inspection of Pharmaceutical and Medical Devices", the Henan Provincial Drug Administration organized a total of 19 unannounced inspections of medical device operating companies.
In accordance with the requirements of medical device operation quality management specifications and supporting regulations and documents, the company focused on the implementation of the main responsibility of quality and safety, product quality and safety management, and conducted a comprehensive inspection of key links such as procurement, acceptance, storage, sales and product traceability. The inspection found that some companies have problems such as incomplete medical device quality management systems, inadequate procurement and acceptance management, and irregular documentation.
For the problems and clues found in the inspection, the inspection team has been handed over to the local supervisory authority of the medical device business enterprise to conduct investigations in accordance with the "Regulations on Medical Device Supervision and Administration", "Medical Device Operation Supervision and Administration Measures", "Medical Device Operation Quality Management Regulations" and other relevant regulations ,deal with. At the same time, urge enterprises to rectify existing problems.
The rectification of unannounced inspections of medical device operating companies in the second half of 2019 (see attachment) is hereby notified.
Source: Henan Traffic Radio 1041
Scan the QR code to read on your phone

Add: No. 206, Xingang Avenue, Airport
District, Zhengzhou
Tel: +86-0371-55285017
Mailbox: kangshengjiutai@kshealthy.com
CopyRight © Zhengzhou Kangsheng Jiutai Medical Equipment Co., Ltd.
This site already supports IPv6



